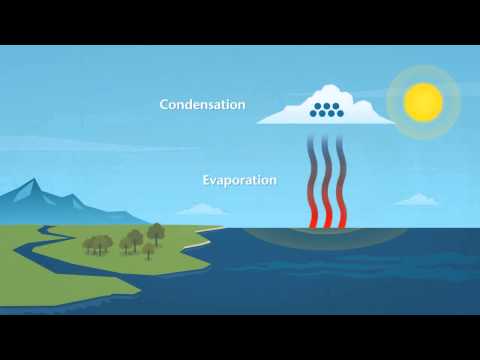
Liquid water is essential for all life on Earth but it is also vitally important in the gaseous form (steam) or as water vapour.
Many compounds can exist on Earth as solids, liquids or gases. By definition, solids have a definite shape and volume and liquids have a definite volume but no definite shape. Gas, however, has neither a definite shape nor a definite volume.
When gas is put into container with a specific volume, it fills the enclosed space. If it is then heated, the gas pressure will rise as the molecules gain energy and move faster, striking the inside area of the container with more force. If more gas is forced into the same space the pressure will also rise as more molecules are striking the same inside area.
Atmospheric gases (including water vapour) are held by the gravitational pull of the Earth and, therefore, have no specific volume. The exact height of the Earth's atmosphere is not fixed, as it can expand and contract based on factors such as temperature, pressure and solar activity.
However, the weight of the gas molecules does exert pressure (on those below) and the pressure decreases with altitude ranging from about 15 pounds/square inch (704 Pascal) at sea level, to near zero at 600 km; the approximate top of the thermosphere.
The heavier gases like oxygen (O2) nitrogen (N), methane (CH4) and carbon dioxide (CO2) tend to be closer to the surface of the Earth. Conversely, lighter gases like hydrogen (H) and helium (He) tend to rise to the top of the atmosphere; where the atoms may be accelerated to escape velocity by impact with high energy solar radiation.
Helium thus tends to drift off into space and may permanently escape from the atmosphere. Helium (He) is a light, rare gas commonly used in balloons, and because it does not combine ready with other elements, probably the only reason that there is any on Earth is that it is constantly created by the slow, spontaneous, radio-active decay of uranium and other naturally radioactive isotopes. Initially, it is trapped inside the fission products but helium atoms are small enough to slowly migrate through solid material and some of these find their way into natural deposits of oil and gas.
Helium can be separated from other gases by cryogenic distillation, where the different gases condense (become liquid) at different low temperatures.
Known reserves continue to decline as once helium escapes to the atmosphere it cannot be economically recovered.
Hydrogen (H) is the lightest gas but it is so reactive it is rarely found naturally as a free element. It is typically a part of many chemical compounds; three of the simplest being with;- carbon in methane (CH4, the main ingredient of natural gas), nitrogen in ammonia (NH3) and oxygen in water (H2O).

YOU ARE READING
Atmospheric
Non-FictionThe eruption of Tonga's underwater volcano, in January 2022, vaporized 150 million tons of seawater and shot a geyser of super heated steam through the stratosphere. The water vapour, a strong greenhouse gas, may increase global temperatures for at...